Autoclaved aerated concrete (AAC, Aircrete)
Autoclaved aerated concrete is a versatile lightweight construction material and usually used as blocks. Compared with normal (ie: “dense” concrete) aircrete has a low density and excellent insulation properties.
The low density is achieved by the formation of air voids to produce a cellular structure. These voids are typically 1mm-5mm across and give the material its characteristic appearance. Blocks typically have strengths ranging from 3-9 Nmm-2 (when tested in accordance with BS EN 771-1:2000). Densities range from about 460 to 750 kg m-3; for comparison, medium density concrete blocks have a typical density range of 1350-1500 kg m-3 and dense concrete blocks a range of 2300-2500 kg m-3.
 Figure 1 Autoclaved aerated concrete block with a sawn surface to show the cellular pore structure (Picture courtesy H+H UK Ltd.)
Figure 1 Autoclaved aerated concrete block with a sawn surface to show the cellular pore structure (Picture courtesy H+H UK Ltd.) Figure 2 Detailed view of cellular pore structure in an aircrete block.
Figure 2 Detailed view of cellular pore structure in an aircrete block.Autoclaved aerated concrete blocks are excellent thermal insulators and are typically used to form the inner leaf of a cavity wall. They are also used in the outer leaf, when they are usually rendered, and in foundations. It is possible to construct virtually an entire house from autoclaved aerated concrete, including walls, floors - using reinforced aircrete beams, ceilings and the roof. Autoclaved aerated concrete is easily cut to any required shape.
Aircrete also has good acoustic properties and it is durable, with good resistance to sulfate attack and to damage by fire and frost.
Production
Autoclaved aerated concrete is cured in an autoclave - a large pressure vessel. In aircrete production the autoclave is normally a steel tube some 3 metres in diameter and 45 metres long. Steam is fed into the autoclave at high pressure, typically reaching a pressure of 800 kPa and a temperature of 180 °C.
Autoclaved aerated concrete can be produced using a wide range of cementitous materials, commonly:
- Portland cement, lime and pulverised fuel ash (PFA, fly ash)
or
- Portland cement, lime and fine silica sand. The sand is usually milled to achieve adequate fineness.
A small amount of anhydrite or gypsum is also often added.
Autoclaved
aerated concrete is quite different from dense concrete (ie: “normal
concrete”) in both the way it is produced and in the composition of the
final product.
Dense concrete is typically a mixture of cement
and water, often with slag or PFA, and fine and coarse aggregate. It
gains strength as the cement hydrates, reaching 50% of its final
strength after perhaps about 2 days and most of its final strength after
a month.
In contrast, autoclaved aerated concrete is of much
lower density than dense concrete. The chemical reactions forming the
hydration products go virtually to completion during autoclaving and so
when removed from the autoclave and cooled, the blocks are ready for
use.
Autoclaved aerated concrete does not contain any aggregate;
all the main mix components are reactive, even milled sand where it is
used. The sand, inert when used in dense concrete, behaves as a pozzolan
in the autoclave due to the high temperature and pressure.
The
autoclaved aerated concrete production process differs slightly between
individual production plants but the principles are similar. We will
assume a mix that contains cement, lime and sand; these are mixed to
form a slurry. Also present in the slurry is fine aluminum powder - this
is added to produce the cellular structure. The density of the final
block can be varied by changing the amount of aluminum powder in the
mix.
The slurry is poured into molds that resemble small railway
wagons with drop-down sides. Over a period of several hours, two
processes occur simultaneously:
The cement hydrates normally to
produce ettringite and calcium silicate hydrates and the mix gradually
stiffens to form what is termed a "green cake".
The green cake
rises in the mold due to the evolution of hydrogen gas formed from the
reaction between the fine aluminum particles and the alkaline liquid.
These gas bubbles give the material its cellular structure.
 Figure 3 Slurry being poured into molds (Picture courtesy H+H UK Ltd.)
Figure 3 Slurry being poured into molds (Picture courtesy H+H UK Ltd.) Figure 4 Green cake rising in mold (Picture courtesy H+H UK Ltd.)
Figure 4 Green cake rising in mold (Picture courtesy H+H UK Ltd.)At the risk of incurring the ire of aircrete manufacturers, it could be said that there are parallels between autoclaved aerated concrete production and bread-making. In bread, the dough contains yeast and is mixed, then left to rise as the yeast converts sugars to carbon dioxide.
The dough must have the right consistency; too hard and the bubbles of carbon dioxide cannot 'stretch' the dough to make it rise, but if the dough is too sloppy, the carbon dioxide bubbles rise to the surface and are lost and the dough collapses. With the right consistency, the dough is sufficiently elastic to stretch and expand, but strong enough to retain the gas so that the dough does not collapse. When risen, the dough is placed in the oven.
Although a much more complex process, Aircrete production conditions are precisely-controlled for, in part, somewhat similar reasons. The mix proportions and the initial mix temperature must be correct and the aluminum powder must be present in the required amount and with the appropriate reactivity an an alkaline environment. All of the materials be be of suitable fineness. A complicating factor is that the temperature of the green cake increases due to the exothermic reactions as the lime and the cement hydrate, so the reactions proceed faster.
When the cake has risen to the required height, the mold moves along a track to where the cake is cut to the required block size. Depending on the actual production process, the cake may be demolded entirely onto a trolley before cutting, or it may be cut in the mold after the sides are removed.
The cake is cut by passing through a series of cutting wires.
 Figure 5 Green cake being cut by wires (Picture courtesy H+H UK Ltd.)
Figure 5 Green cake being cut by wires (Picture courtesy H+H UK Ltd.)At the cutting stage, the blocks are still green - only a few hours has passed since the mix was poured into the mold and they are soft and easily damaged. However, if they are too soft, the cut blocks may either fall apart or stick together; if they are too hard, the wires will not cut them - here too, the process has to be carefully controlled to achieve the necessary consistency.
The cut blocks are then loaded into the autoclave. It takes a couple of hours for the autoclave to reach maximum temperature and pressure, which is held for perhaps 8-10 hours, or longer for high density/high strength aircrete.
 Figure 6 "Green" blocks being loaded into an autoclave (Picture courtesy H+H UK Ltd.)
Figure 6 "Green" blocks being loaded into an autoclave (Picture courtesy H+H UK Ltd.)When removed from the autoclave and cooled, the blocks have achieved their full strength and are packed ready for transport.
AAC Composition
The essence of aircrete production is that lime from the cement and lime in the mix reacts with silica to form 1.1 nm tobermorite (Figure 7).
NB: Cement chemistry notation is used below. If you are not familiar with this, see our cement chemistry notation page.
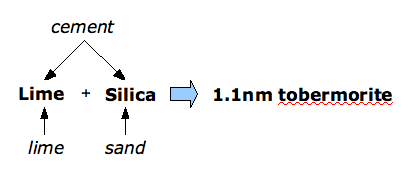 Figure 7 Components and products in aircrete production.
Figure 7 Components and products in aircrete production.During the green stage, the cement is hydrating at normal temperatures
and the hydration products are initially similar to those in dense
concrete - C-S-H, CH and ettringite and/or monosulfate. After
autoclaving, tobermorite is normally the principal final reaction
product due to the high temperature and pressure.
Small amounts
of other hydrated phases will also be present in the final product.
Additionally, hydrated phases form in the autoclave as intermediate
products, principally C-S-H(I). This is a more crystalline form of
calcium silicate hydrate than occurs in dense concrete; it can have a
ratio of calcium to silicon of (0.8<Ca/Si<1.5) but 0.8 to 1.0 is
desirable as this ratio facilitates the formation of 1.1 nm tobermorite.
The
compositions of the hydration products in aircrete are therefore quite
different from those in dense concrete cured at normal temperatures (ie:
calcium silicate hydrate (C-S-H), calcium hydroxide (CH), ettringite
and monosulfate. See the “hydration” page for more information).
Looking
at this in a little more detail from when the green blocks enter the
autoclave, the main reactions that occur are broadly as follows:
- Over 2 hours or so, as the pressure and temperature increase, the normal cement hydration products that formed in the green state progressively disappear and the sand becomes reactive.
- C-S-H(I) forms, partly from silica derived from the sand.
- As more sand reacts, calcium hydroxide from the lime and from cement hydration is gradually used up by continued formation of C-S-H(I).
- With continued autoclaving, 1.1 nm tobermorite starts to crystallize from the C-S-H(I); the total proportion of C-S-H(I) declines and that of 1.1 nm tobermorite gradually increases. C-S-H(I) is therefore mainly an intermediate compound.
The final hydration products are then principally:
- 1.1nm tobermorite
- Possibly some residual C-S-H(I)
- Hydrogarnet
Unreacted sand is likely to remain in the final product. There may also be some residual calcium hydroxide if insufficient silica has reacted and some residual anhydrite and/or hydroxyl-ellestadite if anhydrite was present in the mix.
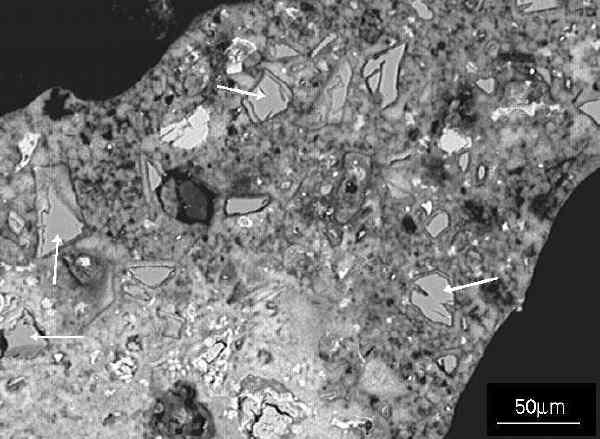 Figure 8 SEM image of polished section showing a detail - a cell wall - of a block made with cement, lime and sand mix.
Figure 8 SEM image of polished section showing a detail - a cell wall - of a block made with cement, lime and sand mix.In Figure 8, some residual unreacted sand particles remain (examples arrowed), often with rims of hydration product showing the size of the original particle. Most of the matrix is composed of tobermorite. Black areas at top left and bottom right are epoxy resin used in preparing the polished section filling air voids (air cells).
The objective is to react sufficient silica from the sand to form tobermorite from the available lime supplied by the lime and cement. This will depend on a range of factors, including the inherent reactivities of the materials, their fineness (especially the sand), and the temperature and pressure. If the autoclaving time is too short, the tobermorite content will not be maximised and some unreacted calcium hydroxide will remain and block strengths will be then less than optimum. If the autoclaving time is too long, other hydration products may form which may also be detrimental to strength and an unnecessary energy cost will be incurred.
There are different forms of tobermorite: 1.1 nm tobermorite and 1.4 nm tobermorite. Also, there are different types of 1.1 nm tobermorite and these behave differently when heated. Their crystal structure is that of layered sheets, with water molecules between the layers - on heating, the inter-layer water is lost; as a result, some 1.1 nm tobermorites shrink (a process known as lattice shrinkage) but some don’t.
1.4 nm tobermorite (C5S6H9) - forms at room temperature and is found as a natural mineral. It decomposes at 55 °C to 1.1 nm tobermorite and so is not found in AAC.
Calcium silicate hydrate compositions in AAC
- 1.1 nm tobermorite (C5S6H5) is usually the main hydration product in AAC where cement, lime and sand are used
- C-S-H(I) - more crystalline than C-S-H in dense concrete, typically 0.8<Ca/Si<1.0
- Xonotlite (C6S6H) - forms with longer autoclaving times, or higher temperatures
Other hydrothermally-formed minerals:
- Gyrolite (C2S3H2) - not normally found in AAC
- Jennite (C9S6H11) occurs as a natural mineral; not found in AAC
- C-S-H(II) - Ca/Si≈ 2.0. Does not occur in AAC
- C2SH (α-C2S hydrate) can occur in autoclaved products but is undesirable
- Hydroxyl-ellestadite (C10S3.3SO3.H2O) - may be found in AAC; also occurs at the cooler end of cement kilns
Environmental benefits of Autoclaved Aerated Concrete
The use of autoclaved aerated concrete has a range of environmental benefits:
Insulation:
most obviously, the insulation properties of aircrete will reduce the
heating costs of buildings constructed with autoclaved aerated concrete,
with consequent fuel savings over the lifetime of the building.
Materials:
lime is one of the principal mix components and requires less energy to
produce than Portland cement, which is fired at higher temperatures.
Sand requires only milling before use, not heating, and PFA is a
by-product from electricity generation. NB: lime may require less energy
to manufacture compared with Portland cement but more CO2 is produced
per tonne (cement approx. 800-900 kg CO2/tonne compared to lime at 1000
kg CO2 per tonne).
Carbonation: less obviously, the cellular
structure of aircrete gives it a very high surface area. Over time, much
of the material is likely to carbonate, largely offsetting the carbon
dioxide produced in the manufacture of the lime and cement due to the
calcining of limestone.
Get a Better Understanding of Cement
Articles like this one can provide a lot of useful material. However, reading an article or two is not really the best way to get a clear picture of a complex material like cement. To get a more complete and integrated understanding of cement and concrete, do have a look at the Understanding Cement book or ebook.
This easy-to-read and concise book contains much more detail on concrete chemistry and deleterious processes in concrete compared with the website.
For example, it has about two-and-a-half times as much on ASR, one-and-a-half times as much on sulfate attack and nearly three times as much on carbonation. It has sections on alkali-carbonate reaction, frost (freeze-thaw) damage, steel corrosion, leaching and efflorescence on masonry. It also has about four-and-a-half times as much on cement hydration (comparisons based on word count).
Click here for more information
Check the Article Directory for more articles on this or related topics