Portland cement clinker - overview
Portland cement clinker is a dark grey nodular material made by heating ground limestone and clay at a temperature of about 1400 °C - 1500 °C. The nodules are ground up to a fine powder to produce cement, with a small amount of gypsum added to control the setting properties.
This page gives a thumbnail sketch. For more information see the following pages, or better still, the Understanding Cement book - links at the bottom of this page.
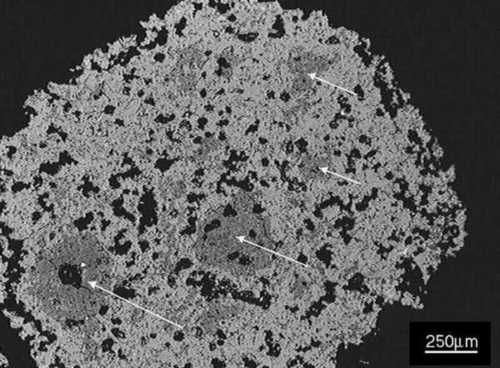 Polished section of nodule (scanning electron microscope image).
Polished section of nodule (scanning electron microscope image).In the above image of a whole nodule in polished section, the nodule has a high alite content and so most of it consists of alite (light gray). Some clusters of belite are visible (arrowed). Aluminate and ferrite phases are present but not visible at this relatively low magnification.
Nodules range in size from 1mm to 25mm or more and are composed mainly of calcium silicates, typically 70%-80%. The strength of concrete is mainly due to the reaction of these calcium silicates with water.
Portland cement clinker contains four principal minerals:
- Alite: approximately tricalcium silicate (typically about 65% of the total)
- Belite: approximately dicalcium silicate (typically about 15% of the total)
- Aluminate: very approximately tricalcium aluminate (typically about 7% of the total)
- Ferrite: very approximately tetracalcium aluminoferrite (typically about 8% of the total)
The balance is made up of alkali sulfates and minor impurities. The typical mineral contents shown are subject to wide variation.
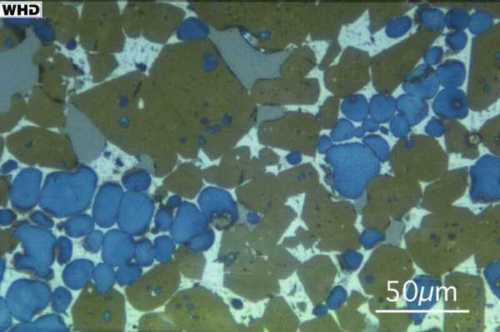 Polished, etched section of a nodule - optical microscope image.
Polished, etched section of a nodule - optical microscope image.In the polished section image above, brown crystals are alite, blue crystals are belite, bright interstitial material is mainly ferrite, with small dark inclusions of aluminate. Gray regions are the epoxy resin used to make the specimen. NB: Alite is not actually brown and belite is not actually blue - they appear brown and blue here because the polished section has been etched with hydrofluoric acid to show the crystals more clearly.
Clinker Composition
In cement chemistry, chemical analyses are normally given in oxide form - a typical example of an analysis follows:
Typical clinker analysis (oxide weight%). |
||||||||||
SiO2 |
Al2O3 |
Fe2O3 |
CaO |
MgO |
K2O |
Na2O |
SO3 |
LOI |
IR |
Total |
21.5 |
5.2 |
2.8 |
66.6 |
1.0 |
0.6 |
0.2 |
1.0 |
1.5 |
0.5 |
98.9 |
|
Free lime = 1.0% CaO Balance is typically due to small amounts of oxides of titanium, manganese, phosphorus and chromium. |
||||||||||
From the chemical analysis, the quantity of each of the four main minerals can be calculated using the 'Bogue' calculation (click on the link below for more information.)
Get a Better Understanding of Cement
Articles like this one can provide a lot of useful material. However, reading an article or two is perhaps not the best way to get a clear picture of a complex process like cement production. To get a more complete and integrated understanding of how cement is made, do have a look at the Understanding Cement book or ebook. This easy-to-read and concise book also contains much more detail on concrete chemistry and deleterious processes in concrete compared with the website.
Click here for more information
You are in: Portland cement clinker - overview
The following pages have more details on chemistry and composition, reactions in the kiln and on cement milling:
Cement notation
/
Clinker compositional parameters
/
Bogue calculation
/
Combinability
/
Reactions in the kiln
/
Cement milling
Check the Article Directory for more articles on this or related topics