Clinker: Cement chemistry notation
Cement chemists use a form of notation which, at first sight - and maybe second or third sight - may seem a little odd.
Oxides are referred to by their first letter: 'C' represents CaO; 'M' is MgO and so on, for all the oxides likely to be encountered in cementitious systems, as shown below.
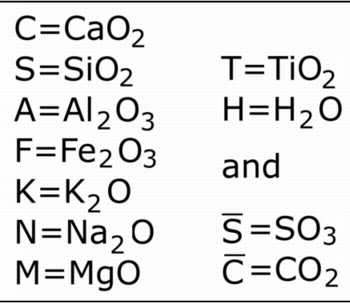
"Normal chemists" who are unfamiliar with this notation may find it strange to use "C" to represent calcium oxide, rather than carbon, but there is a point to all this - it shortens what are otherwise very long names, for example:
Alite: Ca3SiO5 in terms of its oxides is 3CaO.SiO2. The CaO term is shortened to C and the SiO2 to S. The compound thus becomes C3S.
Belite: Similarly, Ca2SiO4 is 2CaO.SiO2, which is shortened to C2S.
Tricalcium aluminate: Ca3Al2O6 is 3CaO.Al2O3. The Al2O3 term is shortened to A and the compound becomes C3A.
Tetracalcium aluminoferrite: 2(Ca2AlFeO5) is 4CaO.Al2O3.Fe2O3. Fe2O3 is shortened to F and the compound becomes C4AF.
(With long names like this last one, the need for a shorthand description is all-too clear.)
In other words, for each of the clinker main minerals, we now have at least three possible descriptions, as below, as well as the full chemical formulae for the pure compounds:
- Alite or tricalcium silicate or C3S
- Belite, or dicalcium silicate or C2S
- Tricalcium aluminate (or the 'aluminate phase') or C3A
- Calcium alumino-ferrite (or the ‘ferrite’ phase) or tetracalcium aluminoferrite or C4AF
Although strictly, these do not mean the same thing, they are frequently used indiscriminately. This lax use means that names like ‘C3A’ should not usually be taken to signify a definite composition in the sense of a pure compound, unless this is indicated by the context.
To take another example, strictly speaking tricalcium silicate is a pure compound, while alite is a mineral composed largely of tricalcium silicate but also with a significant quantity of impurities, mainly magnesium, iron and aluminium.
The symbols for carbonate and sulfate are referred to as 'C bar' and 'S bar,' for obvious reasons. These last two are used less than they once were, perhaps because they are tricky to write using a word processor.
This notation is widely used in cement scientific literature and also in analyses and other information provided by cement manufacturers.
Get a Better Understanding of Cement
Articles like this one can provide a lot of useful material. However, reading an article or two is perhaps not the best way to get a clear picture of a complex process like cement production. To get a more complete and integrated understanding of how cement is made, do have a look at the Understanding Cement book or ebook. This easy-to-read and concise book also contains much more detail on concrete chemistry and deleterious processes in concrete compared with the website.
Click here for more information
You are in: Cement chemistry notation
The following pages have more details on chemistry and composition, reactions in the kiln and on cement milling:
Cement notation
/
Clinker compositional parameters
/
Bogue calculation
/
Combinability
/
Reactions in the kiln
/
Cement milling
Check the Article Directory for more articles on this or related topics