Alkali-silica reaction in concrete
Alkali-silica reaction (ASR) can cause serious expansion and cracking in concrete, resulting in major structural problems and sometimes necessitating demolition. This is a short introduction to ASR - for more information, see the Understanding Cement book/ebook.
Cause of alkali-silica reaction
ASR is the most common form of alkali-aggregate reaction (AAR) in concrete; the other, much less common, form is alkali-carbonate reaction (ACR). ASR and ACR are therefore both subsets of AAR.
ASR is
caused by a reaction between the hydroxyl ions in the alkaline cement
pore solution in the concrete and reactive forms of silica in the
aggregate (eg: chert, quartzite, opal, strained quartz crystals).
A
gel is produced, which increases in volume by taking up water and so
exerts an expansive pressure, resulting in failure of the concrete. In
unrestrained concrete (that is, without any reinforcement), ASR causes
characteristic 'map cracking' or 'Isle of Man cracking'.
Identification of alkali-silica reaction
Gel may be present in cracks and within aggregate particles. The best technique for the identification of ASR is the examination of concrete in thin section, using a petrographic microscope. Alternatively, polished sections of concrete can be examined by scanning electron microscopy (SEM); this has the advantage that the gel can be analysed using X-ray microanalysis in order to confirm the identification beyond any doubt.
 Figure 1 Concrete thin-section, viewed with a petrographic microscope, showing a chert aggregate particle (at the right of the image) from which alkali-silica gel has extruded into adjacent cracks.
Figure 1 Concrete thin-section, viewed with a petrographic microscope, showing a chert aggregate particle (at the right of the image) from which alkali-silica gel has extruded into adjacent cracks.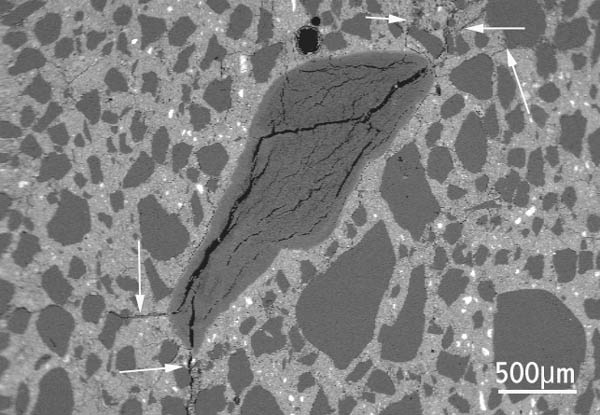 Figure 2 Polished section of concrete, scanning electron microscope image: chert aggregate particle with internal cracks due to ASR extending from the aggregate into the nearby concrete (arrowed).
Figure 2 Polished section of concrete, scanning electron microscope image: chert aggregate particle with internal cracks due to ASR extending from the aggregate into the nearby concrete (arrowed). Figure 3 Detail of chert particle in previous image, showing alkali-silica gel extruded into cracks within the concrete. Ettringite is also present within some cracks.
Figure 3 Detail of chert particle in previous image, showing alkali-silica gel extruded into cracks within the concrete. Ettringite is also present within some cracks.The conditions required for ASR to occur are:
- A sufficiently high alkali content of the cement (or alkali fromother sources)
- A reactive aggregate, such as chert
- Water - ASR will not occur if there is no available water in the concrete, since alkali-silica gel formation requires water
The use of pozzolans in the concrete mix as a partial cement replacement can reduce the likelihood of ASR occurring as they reduce the alkalinity of the pore fluid.
With some aggregates, expansion due to ASR increases in proportion with the amount of reactive aggregate in the concrete. Other aggregates show what is called a “pessimum” effect; if the proportion of reactive aggregate in test mixes is varied, while other factors are kept constant, maximum concrete expansion occurs at a particular aggregate content. Higher or lower proportions of reactive aggregate will give a lower expansion.
The process of ASR is believed to be in many respects similar to the pozzolanic reaction, such as occurs normally in concrete containing pulverised fuel ash (PFA), for example. However, there is an important difference. In the pozzolanic reaction small pozzolanic particles are reacting in a Ca-rich environment, while ASR occurs in mature concrete and involves larger particles of aggregate.
The pozzolanic reaction mechanism is believed to be a process in which silicate anions are detached from the reactive aggregate by hydroxyl ions in the pore fluid. Sodium and potassium ions are the ions most readily-available to balance the silicate anions and an alkali-silicate gel is formed. This can take up (imbibe) water and is mobile. The alkali-silicate gel is unstable in the presence of calcium, and calcium silicate hydrate (C-S-H) is formed.
In the pozzolanic reaction where a pozzolan is used as a partial cement replacement, the particles are small. As there is much calcium available in young concrete, the alkali-silicate gel forms in a thin layer around the pozzolanic particle and quickly converts to C-S-H. No expansion results.
In the case of ASR, the reaction usually occurs much later, possibly years after the concrete was placed. Large aggregate particles (large, that is, compared with cement-sized pozzolan) generate a significant volume of gel which then takes up water and expands within the hardened, mature concrete.
Because the concrete is mature, calcium availability is limited as most of the calcium is bound up in stable solid phases. The rate of supply of calcium is therefore insufficient to convert the gel quickly to C-S-H. Expansion of the gel as water is taken up, may result in damage to the surrounding concrete. Over time, the gel slowly does take up calcium; eventually the composition of the alkali-silica gel may become very similar to that of the calcium silicate hydrate in the cement paste (see Figure 4). By then, though, the damage to the concrete may have already been done.
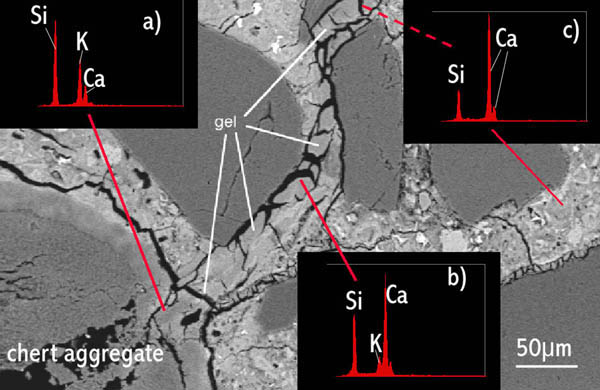 Figure 4 Same image as in Figure 3, with X-ray spectra superimposed showing how alkali-silica gel composition changes with distance from the source, and time. See text.
Figure 4 Same image as in Figure 3, with X-ray spectra superimposed showing how alkali-silica gel composition changes with distance from the source, and time. See text.The composition of the gel extruded from the aggregate changes with time, becoming more similar to the surrounding calcium silicate hydrates. In Figure 4 at a) the gel spectrum shows large peaks due to silicon and potassium (the alkali) and only a very weak peak due to calcium. At b) the calcium peak has become much stronger and the potassium peak much weaker. At c) the potassium peak has disappeared entirely and the gel has approximately the same composition as the normal calcium silicate hydrate comprising the bulk of the cement paste. Clearly, the gel is older with increasing distance from the aggregate particle in which it originated - the 'oldest' gel has had more time in which to take up calcium from the surrounding paste, and has now become calcium silicate hydrate.
Check Out the Understanding Cement Resources!
We have some great training and reference resources. Some are free and some are paid-for.
The paid-for resources are unique to Understanding Cement and proceeds from these sales contribute to the costs of operating and developing this website - see our bookstore here.
The free resources are available on the Cembytes Resources Page. Currently, they include:
Ebook: "Low Concrete Strength? Ten Potential Cement-Related Causes": this illustrated ebook is a checklist of some of the main causes of cement-related low strength in concrete or mortar.
Cement glossary: glossary of over 100 cement-related definitions and chemical formulae.
Screensaver/desktop images: six microscope images of clinker and concrete that you can use as desktop/tablet or screensaver images, or anything else you want.


Subscribers to Cembytes, our free Understanding Cement Newsletter, can download these free resources from the Cembytes Resources Page.
If you are not already a subscriber, just sign up using the box below and you can be downloading the ebook, glossary and photos within a minute or two. Make sure you bookmark the resources page so you can get back to it later - there are no links to it from the website navigation as it is for Cembytes subscribers only!
If you are already a subscriber to Cembytes, you can access the Cembytes Resources Page directly if you have bookmarked it. However, if you have forgotten how to reach the Resources page, you can easily get back to it. Just enter your email in the signup box below and if you are on the subscriber list you will see a link to the Resources Page.
(Note: we currently have two Cembytes subscriber lists, and old one and a new one, and we are gradually transferring to the new one. Depending when you subscribed, you will be on one list or the other. If you are on the old list, the signup box won't recognize you because it is only connected to the new list, but that's fine. Just confirm your subscription and you will be on the new list. After you confirm you will receive an email with a link to the Resources Page.)
Get a Better Understanding of Cement
Articles like this one can provide a lot of useful material. However, reading an article or two is not really the best way to get a clear picture of a complex material like cement. To get a more complete and integrated understanding of cement and concrete, do have a look at the Understanding Cement book or ebook.
This easy-to-read and concise book contains much more detail on concrete chemistry and deleterious processes in concrete compared with the website.
For example, it has about two-and-a-half times as much on ASR, one-and-a-half times as much on sulfate attack and nearly three times as much on carbonation. It has sections on alkali-carbonate reaction, frost (freeze-thaw) damage, steel corrosion, leaching and efflorescence on masonry. It also has about four-and-a-half times as much on cement hydration (comparisons based on word count).
Click here for more information
Check the Article Directory for more articles on this or related topics