Cement hydration
By the process of hydration (reaction with water) Portland cement mixed with sand, gravel and water produces the synthetic rock we call concrete. Concrete is as essential a part of the modern world as are electricity or computers.
Other pages on this web site describe how PC is made and what is in it. Here, we will discuss what happens when it is mixed with water.
This page is a short introduction. The Understanding Cement book/ebook contains a more detailed description of the hydration process and the hydration products.
Clinker is anhydrous (without water) having come from a hot kiln. Cement powder is also anhydrous if we ignore the small amount of water in any gypsum added at the clinker grinding stage.
The reaction with water is termed "hydration". This involves many different reactions, often occurring at the same time. As the reactions proceed, the products of the hydration process gradually bond together the individual sand and gravel particles, and other components of the concrete, to form a solid mass.
The hydration process: reactions
In the anhydrous state, four main types of minerals are normally present: alite, belite, aluminate (C3A) and a ferrite phase (C4AF). For more information on the composition of clinker, see the clinker pages. Also present are small amounts of clinker sulfate (sulfates of sodium, potassium and calcium) and also gypsum, which was added when the clinker was ground up to produce the familiar grey powder.
When water is added, the reactions which occur are mostly exothermic, that is, the reactions generate heat. We can get an indication of the rate at which the minerals are reacting by monitoring the rate at which heat is evolved using a technique called conduction calorimetry. An illustrative example of the heat evolution curve produced is shown below.
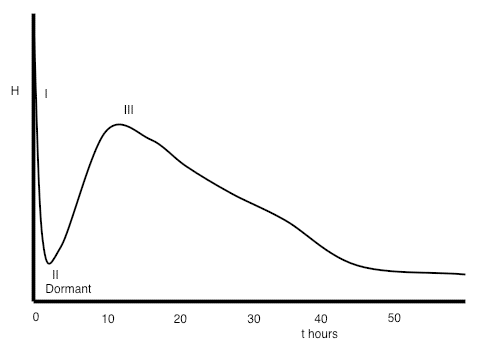
Three principal reactions occur:
Almost immediately on adding water some of the clinker sulphates and gypsum dissolve producing an alkaline, sulfate-rich, solution.
Soon after mixing, the (C3A) phase (the most reactive of the four main clinker minerals) reacts with the water to form an aluminate-rich gel (Stage I on the heat evolution curve above). The gel reacts with sulfate in solution to form small rod-like crystals of ettringite. (C3A) reaction is with water is strongly exothermic but does not last long, typically only a few minutes, and is followed by a period of a few hours of relatively low heat evolution. This is called the dormant, or induction period (Stage II).
The first part of the dormant period, up to perhaps half-way through, corresponds to when concrete can be placed. As the dormant period progresses, the paste becomes too stiff to be workable.
At the end of the dormant period, the alite and belite in the cement start to react, with the formation of calcium silicate hydrate and calcium hydroxide. This corresponds to the main period of hydration (Stage III), during which time concrete strengths increase. The individual grains react from the surface inwards, and the anhydrous particles become smaller. (C3A) hydration also continues, as fresh crystals become accessible to water.
The period of maximum heat evolution occurs typically between about 10 and 20 hours after mixing and then gradually tails off. In a mix containing PC only, most of the strength gain has occurred within about a month. Where PC has been partly-replaced by other materials, such as fly ash, strength growth may occur more slowly and continue for several months or even a year.
Ferrite reaction also starts quickly as water is added, but then slows down, probably because a layer of iron hydroxide gel forms, coating the ferrite and acting as a barrier, preventing further reaction.
Almost everyone interested in cement is also concerned to at least some degree with concrete strength. This ebook describes ten cement-related characteristics of concrete that can potentially cause strengths to be lower than expected. Get the ebook FREE when you sign up to CEMBYTES, our Understanding Cement Newsletter - just click on the ebook image above.
Hydration products
The products of the reaction between cement and water are termed "hydration products." In concrete (or mortar or other cementitious materials) there are typically four main types:
Calcium silicate hydrate: this is the main reaction product and is the main source of concrete strength. It is often abbreviated, using cement chemists' notation, to "C-S-H," the dashes indicating that no strict ratio of SiO2 to CaO is inferred. The Si/Ca ratio is somewhat variable but typically approximately 0.45-0.50 in hydrated Portland cement but up to perhaps about 0.6 if slag or fly ash or microsilica is present, depending on the proportions.
Calcium hydroxide: (or Portlandite)- Ca(OH)2, often abbreviated to 'CH.' CH is formed mainly from alite hydration. Alite has a Ca:Si ratio of 3:1 and C-S-H has a Ca/Si ratio of approximately 2:1, so excess lime is available to produce CH.
AFm and AFt phases: these are two groups of minerals that occur in cement, and elsewhere. One of the most common AFm phases in hydrated cement is monosulfate and by far the most common AFt phase is ettringite. The general definitions of these phases are somewhat technical, but for example, ettringite is an AFt phase because it contains three (t-tri) molecules of anhydrite when written as C3A.3CaSO4.32H2O and monosulfate is an AFm phase because it contains one (m-mono) molecule of anhydrite when written as C3A.CaSO4.12H2O.
The most common AFt and AFm phases in hydrated cement are:
Ettringite: ettringite is present as rod-like crystals in the early stages of reaction or sometimes as massive growths filling pores or cracks in mature concrete or mortar. The chemical formula for ettringite is [Ca3Al(OH)6.12H2O]2.2H2O] or, mixing notations, C3A.3CaSO4.32H2O.
Monosulfate: monosulfate tends to occur in the later stages of hydration, a day or two after mixing. The chemical formula for monosulfate is C3A.CaSO4.12H2O. Note that both ettringite and monosulfate are compounds of C3A, CaSO4 (anhydrite) and water, in different proportions.
Monocarbonate: the presence of fine limestone, whether interground with the cement or present as fine limestone aggregate, is likely to produce monocarbonate (C3A.CaCO3.11H2O) as some of the limestone reacts with the cement pore fluid.
Other AFm phases that may be present are hemicarbonate, hydroxy-AFm and Friedel's salt.
Some important points to note about AFm and AFt phases are that:
- They contain a lot of water, especially AFt - principally ettringite in the context of cement.
- AFm contains a higher ratio of aluminium/calcium compared with AFt.
- The aluminium can be partly-replaced by iron in both AFm and AFt phases.
- The sulfate ion in monosulfate AFm phase can be replaced by other anions; a one-for-one substitution if the anion is doubly-charged (eg: carbonate, CO22-) or one-for-two if the substituent anion is singly-charged (eg: hydroxyl, OH- or chloride, Cl-).
- The sulfate
in ettringite can be replaced by carbonate or, probably, partly replaced
by two hydroxyl ions, although in practice neither of these is often
observed.
In a concrete made from cement containing only clinker
and gypsum, ettringite forms early after the cement and water are mixed, but it is gradually replaced by monosulfate. This is because the ratio of
available alumina to sulfate increases with continued cement hydration;
on first contact with water, most of the sulfate is readily available to
dissolve, but much of the C3A is contained inside cement grains with no
initial access to water. Continued hydration gradually releases alumina
and the proportion of ettringite decreases as that of monosulfate
increases.
If there is eventually more alumina than sulfate
available, all the sulfate will be as monosulfate, with the additional
alumina present as hydroxyl-substituted AFm phase (hydroxy-AFm). If
there is a small excess of sulfate, the cement paste will contain a
mixture of monosulfate and ettringite. With increasing available
sulfate, there will be more ettringite and less monosulfate, and at even
higher levels of sulfate there will be ettringite and gypsum.
If
fine limestone is present, carbonate ions become available as some of
the limestone reacts. The carbonate displaces sulfate or hydroxyl in
AFm; the proportion of monosulfate or hydroxy-AFm therefore decreases as
the proportion of monocarbonate increases. The displaced sulfate
typically combines with remaining monosulfate to form ettringite, but if
any hydroxy-AFm is present, the sulfate will displace the hydroxyl ions
to form more monosulfate. The key here is the balance between available
alumina on the one hand, and carbonate and sulfate on the other.
Hydrogarnet:
hydrogarnet forms mainly as the result of ferrite or C3A hydration.
Hydrogarnets have a range of compositions, of which C3AH6 is the most
common phase forming from normal cement hydration and then only in small
amounts. A wider range of hydrogarnet compositions can be found in
autoclaved cement products.
Check Out the Understanding Cement Resources!
We have some great training and reference resources. Some are free and some are paid-for.
The paid-for resources are unique to Understanding Cement and proceeds from these sales contribute to the costs of operating and developing this website - see our bookstore here.
The free resources are available on the Cembytes Resources Page. Currently, they include:
Ebook: "Low Concrete Strength? Ten Potential Cement-Related Causes": this illustrated ebook is a checklist of some of the main causes of cement-related low strength in concrete or mortar.
Cement glossary: glossary of over 100 cement-related definitions and chemical formulae.
Screensaver/desktop images: six microscope images of clinker and concrete that you can use as desktop/tablet or screensaver images, or anything else you want.


Subscribers to Cembytes, our free Understanding Cement Newsletter, can download these free resources from the Cembytes Resources Page.
If you are not already a subscriber, just sign up using the box below and you can be downloading the ebook, glossary and photos within a minute or two. Make sure you bookmark the resources page so you can get back to it later - there are no links to it from the website navigation as it is for Cembytes subscribers only!
If you are already a subscriber to Cembytes, you can access the Cembytes Resources Page directly if you have bookmarked it. However, if you have forgotten how to reach the Resources page, you can easily get back to it. Just enter your email in the signup box below and if you are on the subscriber list you will see a link to the Resources Page.
(Note: we currently have two Cembytes subscriber lists, and old one and a new one, and we are gradually transferring to the new one. Depending when you subscribed, you will be on one list or the other. If you are on the old list, the signup box won't recognize you because it is only connected to the new list, but that's fine. Just confirm your subscription and you will be on the new list. After you confirm you will receive an email with a link to the Resources Page.)
Get a Better Understanding of Cement
Articles like this one can provide a lot of useful material. However, reading an article or two is not really the best way to get a clear picture of a complex material like cement. To get a more complete and integrated understanding of cement and concrete, do have a look at the Understanding Cement book or ebook.
This easy-to-read and concise book contains much more detail on concrete chemistry and deleterious processes in concrete compared with the website.
For example, it has about two-and-a-half times as much on ASR, one-and-a-half times as much on sulfate attack and nearly three times as much on carbonation. It has sections on alkali-carbonate reaction, frost (freeze-thaw) damage, steel corrosion, leaching and efflorescence on masonry. It also has about four-and-a-half times as much on cement hydration (comparisons based on word count).
Click here for more information
Check the Article Directory for more articles on this or related topics